
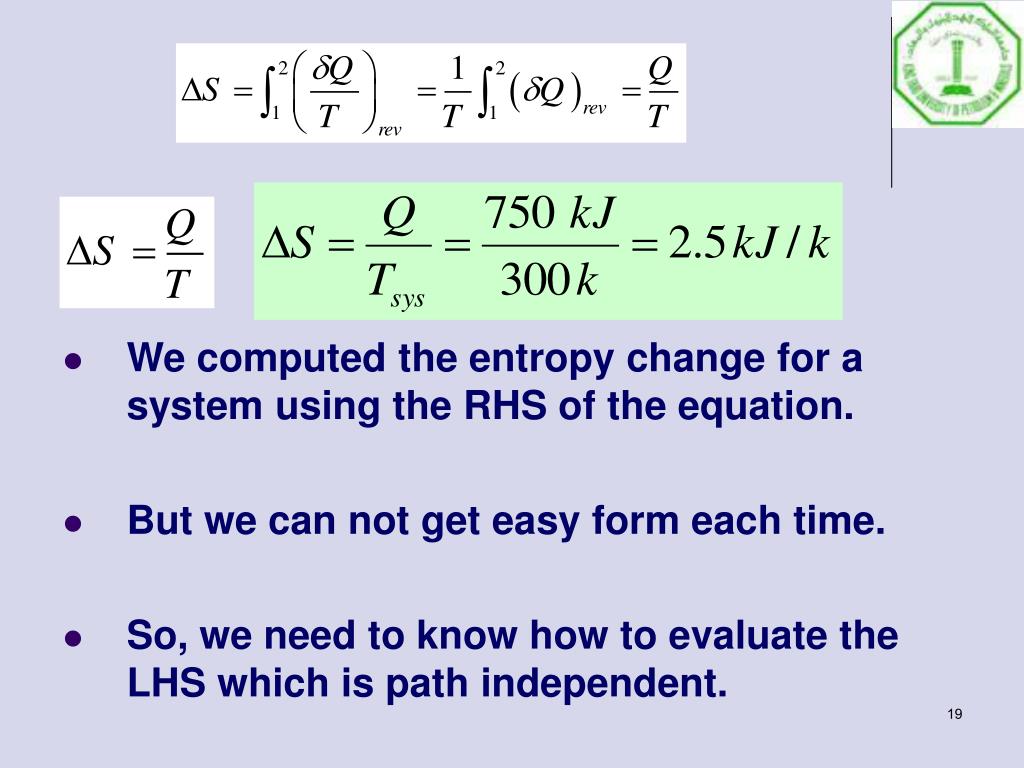
The fundamental form of the rate of increase in entropy in an irreversible process is expressed as the sum of the products of the thermodynamic forces and the flows within the system. Therefore, the value of entropy corresponds to entropies of the real system at a certain time. This assumption enables one to devise an ideal process that would bring the system reversibly to any configuration through which a system passes during an irreversible change. However entropy is determined only by the set of local variables characterizing the state of the system. For a set of specified external variables, we can reach the same internal variables in various ways, both reversible and irreversible. Schrodinger associated the concept of entropy with biological systems and stated that the existence of all living beings is based on entropy export, but it is also closely connected with information processing.Įntropy at any stage of an irreversible process is determined by assuming that the entropy is a unique function of the external and internal variables, regardless the energy and work capacity of the system. The energy of the world remains constant but its usability diminishes with every increase in the worlds' entropy. Entropy is a measure of the work value of the energy contained in the system, and the maximal entropy (thermodynamic equilibrium) means that the energy has zero work value, while low entropy means that the energy has relatively high work value. The directional properties and the increase of entropy in natural adiabatic processes have lead to various interpretations of entropy: Clausius believed that the laws of thermodynamics have a universal validity. Both entropy increase and time are intimately associated with the behavior of natural phenomena, and the fundamental law for closed, adiabatic system, dS/dt > 0, may be regarded as the pointer of the arrow of time. Entropy can be used to distinguish between reversible and irreversible processes. A system and its surrounding create an isolated system where the sum of the entropies of all bodies involving a reversible change remains the same, and increases during irreversible processes. For reversible processes dS is an exact differential of the state function entropy, and the final result of the integration does not depend on the path of the process or on how it is materialized, provided that both the initial and final states are stable equilibrium states.Įntropy of a closed adiabatic system remains the same in a reversible process, and increases during an irreversible process. Entropy is a thermodynamic potential, and is not conserved it gives a quantitative measure of irreversibility. Mathematical statement of the second law is associated with the definition of entropy S, dS = δQ rev / T. Yaşar Demirel, in Nonequilibrium Thermodynamics, 2002 1.


 0 kommentar(er)
0 kommentar(er)
